Neuralink Confirms First Human Brain Chip Implant in January, Eyes Future Clinical Trials
Breaking news in neurotechnology: Neuralink Confirms First Human Brain Chip Implant in January, Eyes Future Clinical Trials This Year. This monumental development, announced by Elon Musk, marks a significant leap forward in the quest to connect human brains directly with computers, promising revolutionary advancements in medical treatment and human interaction.
Neuralink’s Historic First Human Implant
Neuralink, the neurotechnology company founded by Elon Musk, has officially confirmed the successful implantation of its brain chip device into a human patient in January. This procedure represents a critical milestone after years of research and development, moving the company from animal trials to direct human application. The patient is reportedly recovering well, with initial results showing promising neuron spike detection.
The company’s primary objective for this initial phase is to enable individuals with severe neurological conditions, such as paralysis, to control external devices with their thoughts. This initial success sets the stage for a series of future clinical trials, as Neuralink aims to refine its technology and broaden its applications. The long-term vision includes restoring motor function, improving communication for those with locked-in syndrome, and potentially enhancing human cognitive abilities.
The PRIME Study: A Closer Look
The first human trial, known as the PRIME (Precise Robotically Implanted Brain-Computer Interface) Study, focuses on evaluating the safety and initial functionality of Neuralink’s fully implantable, wireless brain-computer interface (BCI). This study targets individuals who have lost the ability to use their hands due to cervical spinal cord injury or amyotrophic lateral sclerosis (ALS).
- Target Patients: Individuals with severe paralysis, particularly those with quadriplegia.
- Primary Goal: To assess the safety of the N1 Link and the surgical robot, and to evaluate the initial functionality of the BCI for controlling external devices.
- Expected Outcomes: Allow participants to control a computer cursor or keyboard using their thoughts.
The PRIME Study is a crucial step in Neuralink’s regulatory pathway, providing essential data for future approvals and wider deployment of their technology. The data gathered from this initial human implant will be instrumental in demonstrating the device’s efficacy and safety to regulatory bodies.
Technological Breakthroughs Behind the Implant
The success of the first human implant by Neuralink is underpinned by several significant technological advancements, particularly in electrode design, surgical robotics, and data processing. These innovations are crucial for creating a safe, effective, and minimally invasive brain-computer interface.
Neuralink’s device, known as the N1 Link, is a small, coin-sized implant designed to be cosmetically invisible once placed in the skull. It contains thousands of ultra-fine threads, each thinner than a human hair, equipped with electrodes capable of detecting neural activity. These threads are meticulously implanted into the brain’s motor cortex, the region responsible for planning and executing voluntary movements.
The R1 Surgical Robot: Precision Implantation
A key component of Neuralink’s strategy is the R1 surgical robot, specifically designed to implant the N1 Link’s delicate threads with unparalleled precision. Manual implantation of such fine electrodes would be nearly impossible and highly risky. The R1 robot uses advanced imaging and navigation systems to avoid blood vessels and minimize tissue damage during the procedure.
- Automated Precision: The R1 robot can implant up to 64 threads per minute, each carrying 1,024 electrodes.
- Minimally Invasive: Designed to reduce surgical risks and recovery time.
- Vascular Avoidance: Uses optical coherence tomography (OCT) to navigate around blood vessels.
This robotic precision is vital for the long-term viability and safety of the implant, ensuring that the electrodes are placed optimally to capture neural signals effectively while minimizing any potential harm to brain tissue.
Ethical Considerations and Regulatory Oversight
As Neuralink pushes the boundaries of neurotechnology, ethical considerations and stringent regulatory oversight become paramount. The prospect of directly interfacing with the human brain raises complex questions about patient safety, data privacy, and the long-term societal impact of such powerful technology.
The U.S. Food and Drug Administration (FDA) plays a critical role in approving such devices for human use. Neuralink received FDA approval for its first-in-human clinical trial in May 2023, a significant hurdle that required extensive preclinical data demonstrating the device’s safety and efficacy in animal studies. This approval signaled the FDA’s confidence in Neuralink’s initial research and development protocols.
Navigating the Ethical Landscape
The ethical framework surrounding BCIs is still evolving. Key areas of concern include:
- Informed Consent: Ensuring participants fully understand the risks and potential benefits of brain implantation.
- Data Security: Protecting highly sensitive neural data from unauthorized access or misuse.
- Cognitive Enhancement: Addressing the implications if the technology advances beyond medical applications to cognitive augmentation.
Neuralink has stated its commitment to addressing these ethical challenges responsibly, emphasizing that its initial focus is on restoring function for those with severe disabilities. Public discourse and ongoing regulatory dialogue will be essential in shaping the responsible development and deployment of this technology.
The Patient Experience: Early Insights
While specific details about the first human patient remain confidential, initial reports from Neuralink indicate a positive recovery post-implantation. Elon Musk confirmed that the patient is doing well and that the device is already detecting neuron spikes, which is a crucial first step in demonstrating its functionality.
The patient experience in these early trials is vital for understanding the real-world implications of brain-computer interfaces. Beyond the technical aspects, factors such as comfort, ease of use, and the psychological impact of having a brain implant are closely monitored. The goal is not just technological success but also improving the quality of life for participants.
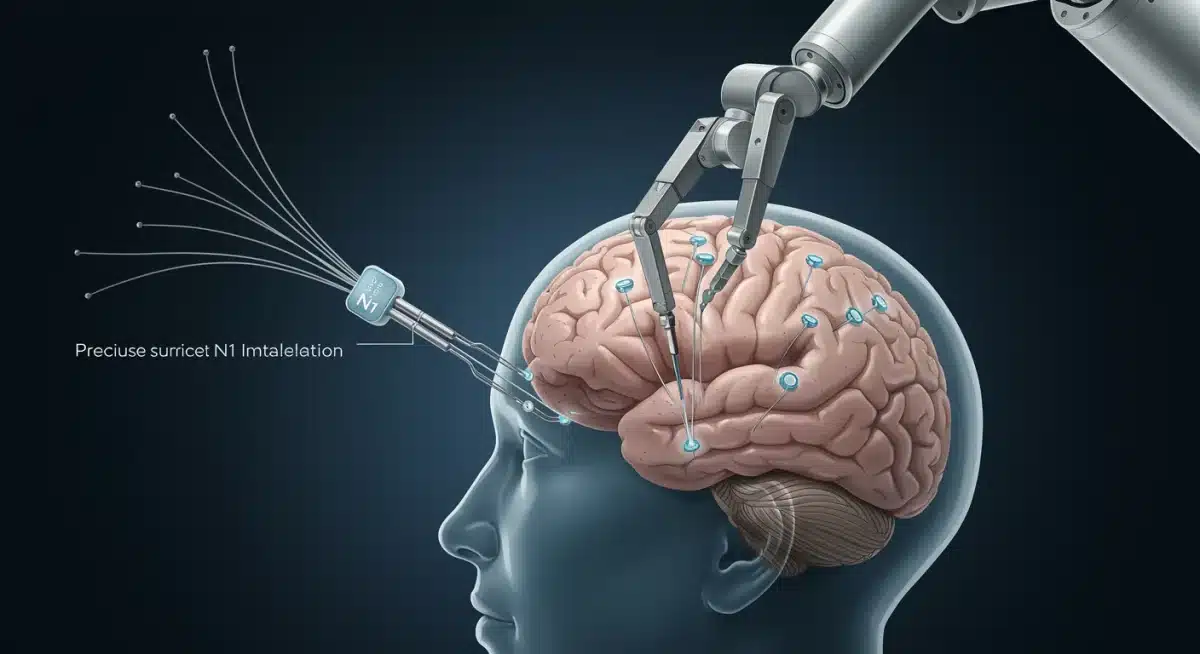
Training and Adaptation
Following the implantation, patients undergo a period of intensive training to learn how to control external devices using their thoughts. This involves mental exercises and feedback mechanisms that help them refine their neural signals to interact with computers effectively. The process is akin to learning a new skill, requiring patience and dedication from the participant.
Early data will focus on:
- Signal Quality: How clearly and consistently the device can read neural activity.
- Control Accuracy: The patient’s ability to precisely manipulate a cursor or other interfaces.
- Patient Feedback: Qualitative assessments of comfort, usability, and overall experience.
These early insights will guide further development, helping Neuralink optimize both the hardware and the software algorithms that translate brain signals into actionable commands.
Future Clinical Trials and Long-Term Vision
With the successful first human implant, Neuralink is now poised to expand its clinical trial efforts throughout the year. The company’s long-term vision extends far beyond simply assisting individuals with paralysis; it encompasses a future where brain-computer interfaces could significantly enhance human capabilities and address a wider range of neurological disorders.
Future trials will likely explore additional applications, including restoring vision for the blind, enabling hearing for the deaf, and even treating conditions like Parkinson’s disease, epilepsy, and depression. The modular nature of Neuralink’s technology suggests that different brain regions could be targeted for various therapeutic outcomes.
Expanding Beyond Medical Applications
While the immediate focus remains on medical applications, Elon Musk has openly discussed the broader implications of Neuralink’s technology, including the potential for cognitive enhancement and a symbiotic relationship between humans and artificial intelligence. This ambitious vision positions Neuralink at the forefront of a new era of human evolution.
- Cognitive Enhancement: Potentially augmenting memory, learning, and communication.
- AI Symbiosis: Creating a direct interface between human consciousness and AI.
- Disease Prevention: Early detection and intervention for neurological conditions.
However, these advanced applications are still many years, if not decades, away and will require extensive research, ethical debate, and regulatory frameworks to ensure responsible development.
Impact on Neurotechnology and Society
The news that Neuralink Confirms First Human Brain Chip Implant in January, Eyes Future Clinical Trials This Year has sent ripples throughout the neurotechnology community and beyond. This development is not just about a single company’s achievement; it represents a significant advancement for the entire field of brain-computer interfaces and holds profound implications for society.
The success of a high-profile company like Neuralink can accelerate research and investment in neurotechnology across the globe. It validates the potential of BCIs to revolutionize medicine and human interaction, encouraging other researchers and companies to push their own boundaries. This increased competition and collaboration could lead to quicker innovations and broader access to these life-changing technologies.
Shaping the Future of Human-Computer Interaction
Beyond medical applications, Neuralink’s work points towards a future where human-computer interaction is seamless and intuitive. Imagine controlling devices, communicating, or even accessing information directly with your thoughts, bypassing traditional interfaces like keyboards or screens. This paradigm shift could redefine how humans live, work, and interact with the digital world.
- Direct Digital Control: Thought-controlled devices, eliminating physical interfaces.
- Enhanced Communication: Telepathy-like communication for those with speech impairments.
- Personalized Healthcare: Real-time monitoring and intervention for neurological health.
However, the societal integration of such technology will require careful consideration of accessibility, equity, and the potential for digital divides. Ensuring that these advancements benefit all of humanity, not just a select few, will be a critical challenge in the years to come.
| Key Point | Brief Description |
|---|---|
| First Human Implant | Neuralink successfully implanted its brain chip into a human patient in January, a major milestone. |
| PRIME Study Focus | The initial trial aims to enable thought-control of devices for individuals with severe paralysis. |
| R1 Surgical Robot | A specialized robot ensures precise and minimally invasive implantation of the N1 Link’s threads. |
| Future Trials & Vision | Neuralink plans expanded trials and envisions broader applications, including cognitive enhancement. |
Frequently Asked Questions About Neuralink’s Human Implant
The primary goal is to enable individuals with severe paralysis to control external devices, such as computer cursors or keyboards, using only their thoughts. This initial trial focuses on assessing the safety and basic functionality of the N1 Link device.
The PRIME Study is designed for individuals who have lost the ability to use their hands due to conditions like cervical spinal cord injury or amyotrophic lateral sclerosis (ALS). Participants must meet specific medical criteria for inclusion in the trial.
The Neuralink chip, called the N1 Link, is implanted into the brain’s motor cortex by a specialized surgical robot, the R1. This robot uses advanced imaging to precisely place thousands of ultra-fine electrode threads, minimizing invasiveness and risk.
Ethical concerns include ensuring robust data privacy and security for neural information, obtaining truly informed consent from participants, and addressing the long-term societal implications if the technology progresses to cognitive enhancement beyond medical necessity.
Beyond treating paralysis, Neuralink envisions restoring vision and hearing, addressing neurological disorders like Parkinson’s, and potentially enabling cognitive enhancement. Ultimately, it aims for a symbiotic relationship between humans and artificial intelligence.
What Happens Next
The successful first human implant by Neuralink signals the start of a decisive phase where real-world data will drive innovation at an accelerated pace. As the company begins continuous monitoring and optimization, much like the technological breakthroughs covered by CNN in their recent report on Neuralink’s early human trials (https://edition.cnn.com/2024/01/30/business/elon-musk-brain-implant-neuralink-intl-hnk), global attention is now shifting from possibility to measurable outcomes.
Over the next few months, updates regarding the patient’s ability to control external systems will serve as key milestones, potentially opening the door to wider clinical inclusion and applications beyond medical rehabilitation. If these early indicators prove successful, Neuralink could rapidly transition from a pioneering experiment to a defining force in neuroprosthetics and human-computer interaction. This transition period will ultimately determine how quickly the technology reaches broader clinical availability — and whether it truly represents the next leap in human augmentation.